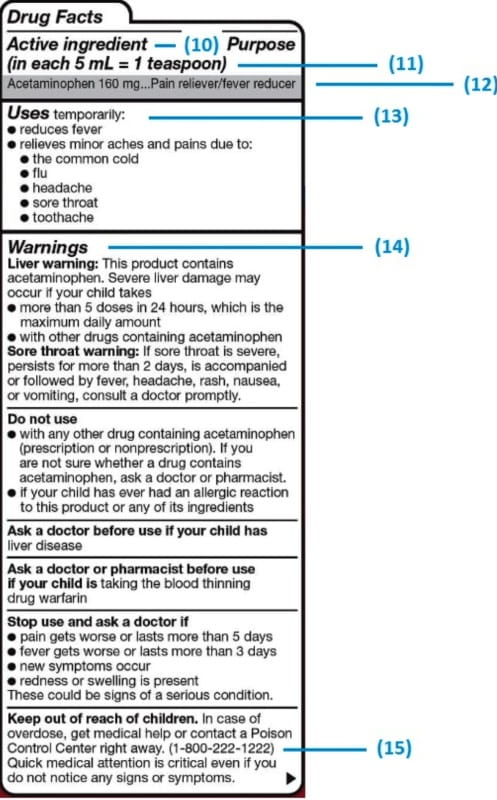
43 medication labels must include
Best practice guidance on the labelling and packaging of ... Where a generic medicine has a company name included as part of the name registered in section 1 of the SmPC the full colour mock-ups should not include ...16 pages How to Read Medication Orders and Drug Labels - Oregon.gov Labels on prescription medications; ... looks different or is an electronic medical order, it must have the following information: • Name of resident;.13 pages
Generic Drugs: Questions & Answers | FDA Mar 16, 2021 · A generic drug is a medication created to be the same as an already marketed brand-name drug in dosage form, safety, strength, route of administration, quality, performance characteristics, and ...
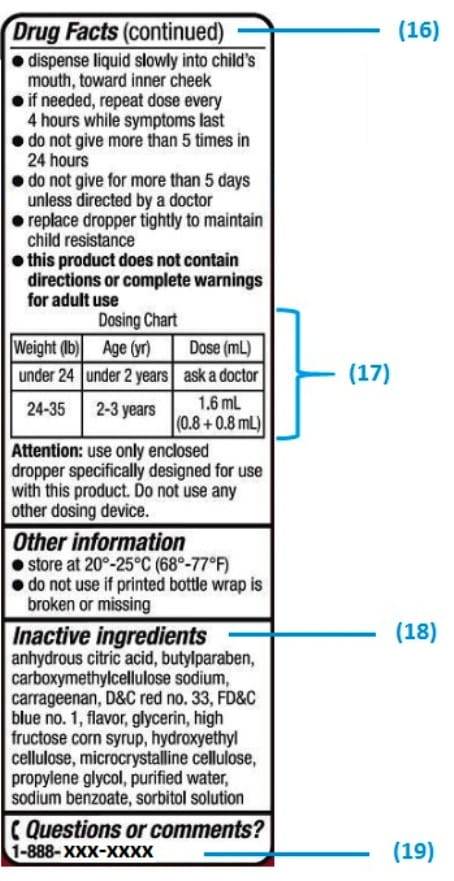
Medication labels must include
Medication Labels 101: Categories, Regulations, and Best ... The basics of medication labels include the drug name, dosage, and directions. Medication labels should always include warnings regarding safety. How to Label a Medical Syringe 14 Mar 2022 — Start with the label design and where the information is printed, especially key data like patient name, drug name, dose instructions, dosage ... What Information Should Be on Drug Labels? - MedicineNet 3 Feb 2022 — Labeling requirements for prescription drugs · Statement of identity · Brand name · Net quantity of contents · Statement of dosage.
Medication labels must include. Medicines: packaging, labelling and patient information leaflets 18 Dec 2014 — Labels must include warnings for safe use of the medicine. All products that contain paracetamol must include statutory warnings. Additional ... NIMH » Mental Health Medications Antipsychotic treatment for older adults necessitates additional care and consideration. The FDA requires that all antipsychotic medication labels include a black-box warning stating that antipsychotics are associated with increased rates of stroke and death in older adults with dementia. Prescription drug - Wikipedia The expiration date is the final day that the manufacturer guarantees the full potency and safety of a medication. Drug expiration dates exist on most medication labels, including prescription, over-the-counter (OTC) and dietary (herbal) supplements. Magnesium - Health Professional Fact Sheet - Magnesium deficiency This is a fact sheet intended for health professionals. For a reader-friendly overview of Magnesium, see our consumer fact sheet on Magnesium.. Introduction. Magnesium, an abundant mineral in the body, is naturally present in many foods, added to other food products, available as a dietary supplement, and present in some medicines (such as antacids and laxatives).
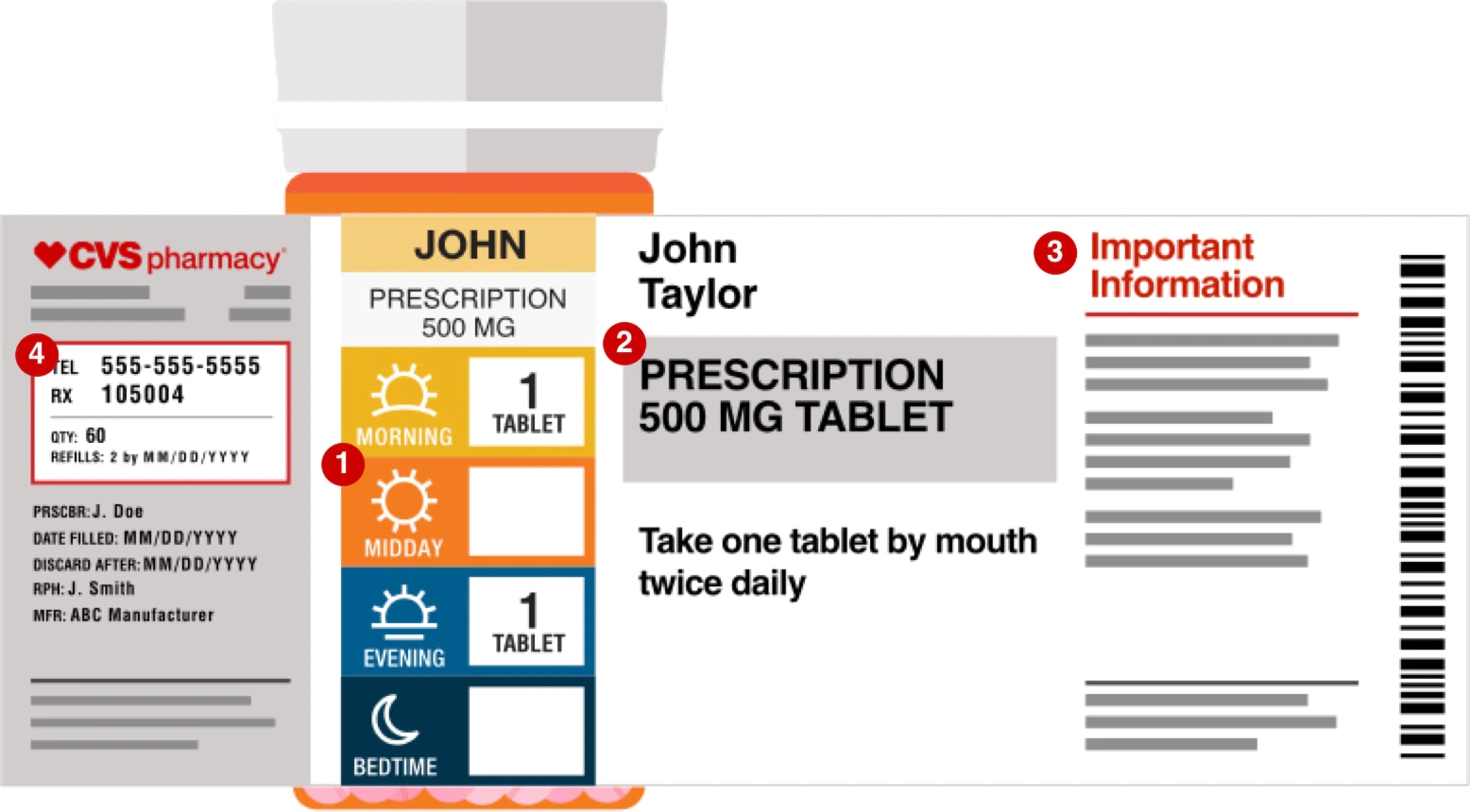
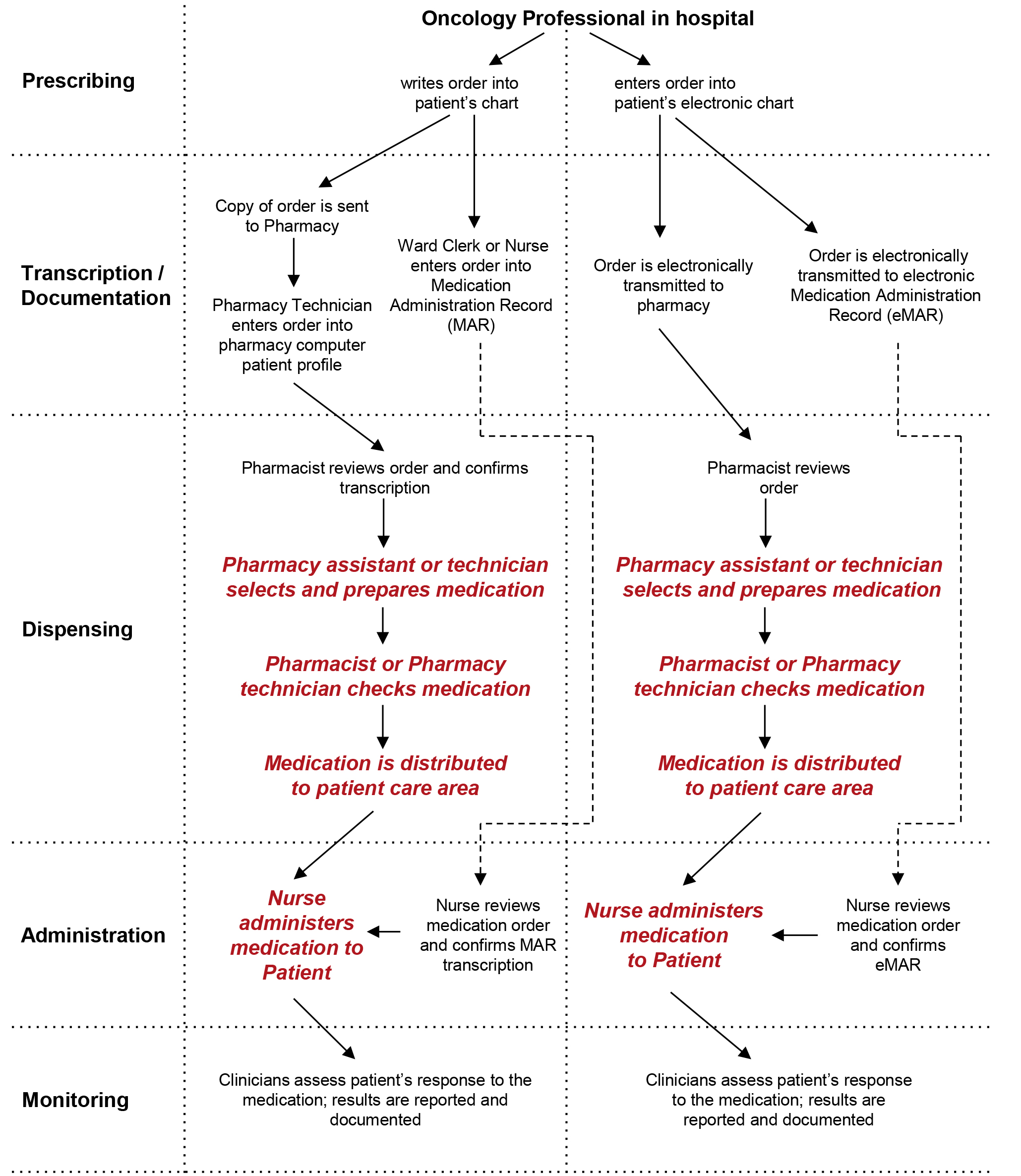
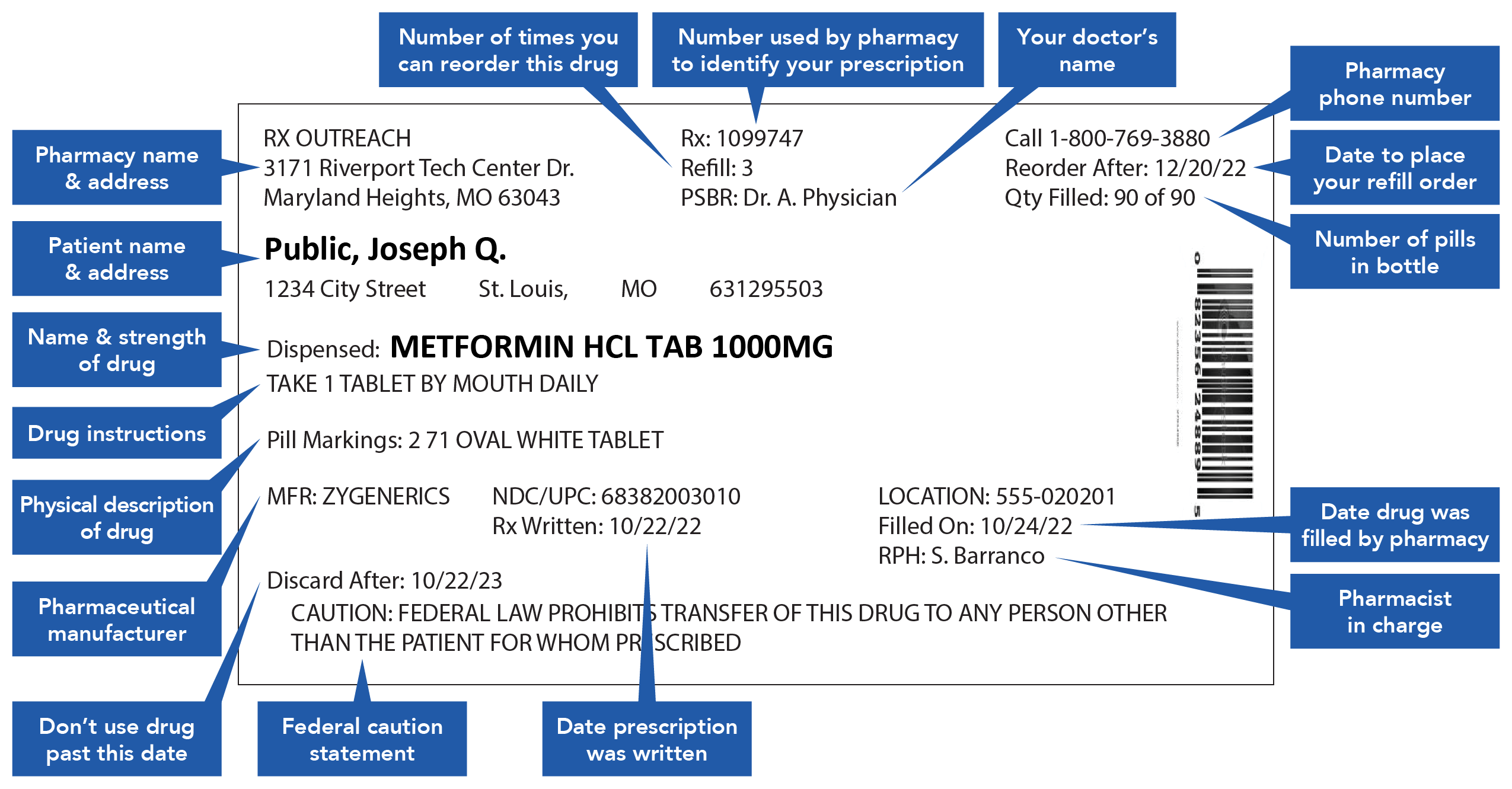
Medication Administration Regulations - ct (3) Monitor and document on an ongoing basis, but at least quarterly, documentation pertaining to the administration of medication. Review shall include, but not be limited to: (a) physician or dentist orders; (b) medication bottle labels and medications listed on the medication record and receipt and distribution form or forms to determine ... Statement on Labeling of Pharmaceuticals for Use in ... Label content: Syringes: The drug's generic name and concentration (in units per mL) should be the most prominent items displayed on the label of each syringe. Report a COVID-19 rapid lateral flow test result - GOV.UK Use this service to report your result to the NHS after using a rapid lateral flow test kit to check if you’re infectious with coronavirus (COVID-19). You cannot use this service to report ... Understanding Prescription Medication Labels - Rx Outreach If it says “three times a day”, take the medication at three intervals, such as morning – afternoon – evening. Always read the warning labels on your prescription (if applies). The warning labels are usually on the side or back and are often separated from the main label. The package insert will describe all side-effects and warnings.
Understanding the Critical Requirements for FDA Drug Labels FDA regulations require that all medication labels include: · Name of Product · Table of Drug Facts · Active Ingredients · Proper Use and Purpose · Warnings ... Drug Labeling - StatPearls - NCBI Bookshelf by MJ Lopez · 2021 · Cited by 5 — Definition/Introduction · Highlights (a concise summary of label information) · Full prescribing Information · Limitations Statement · Product Names. What Information Should Be on Drug Labels? - MedicineNet 3 Feb 2022 — Labeling requirements for prescription drugs · Statement of identity · Brand name · Net quantity of contents · Statement of dosage. How to Label a Medical Syringe 14 Mar 2022 — Start with the label design and where the information is printed, especially key data like patient name, drug name, dose instructions, dosage ...
Medication Labels 101: Categories, Regulations, and Best ... The basics of medication labels include the drug name, dosage, and directions. Medication labels should always include warnings regarding safety.









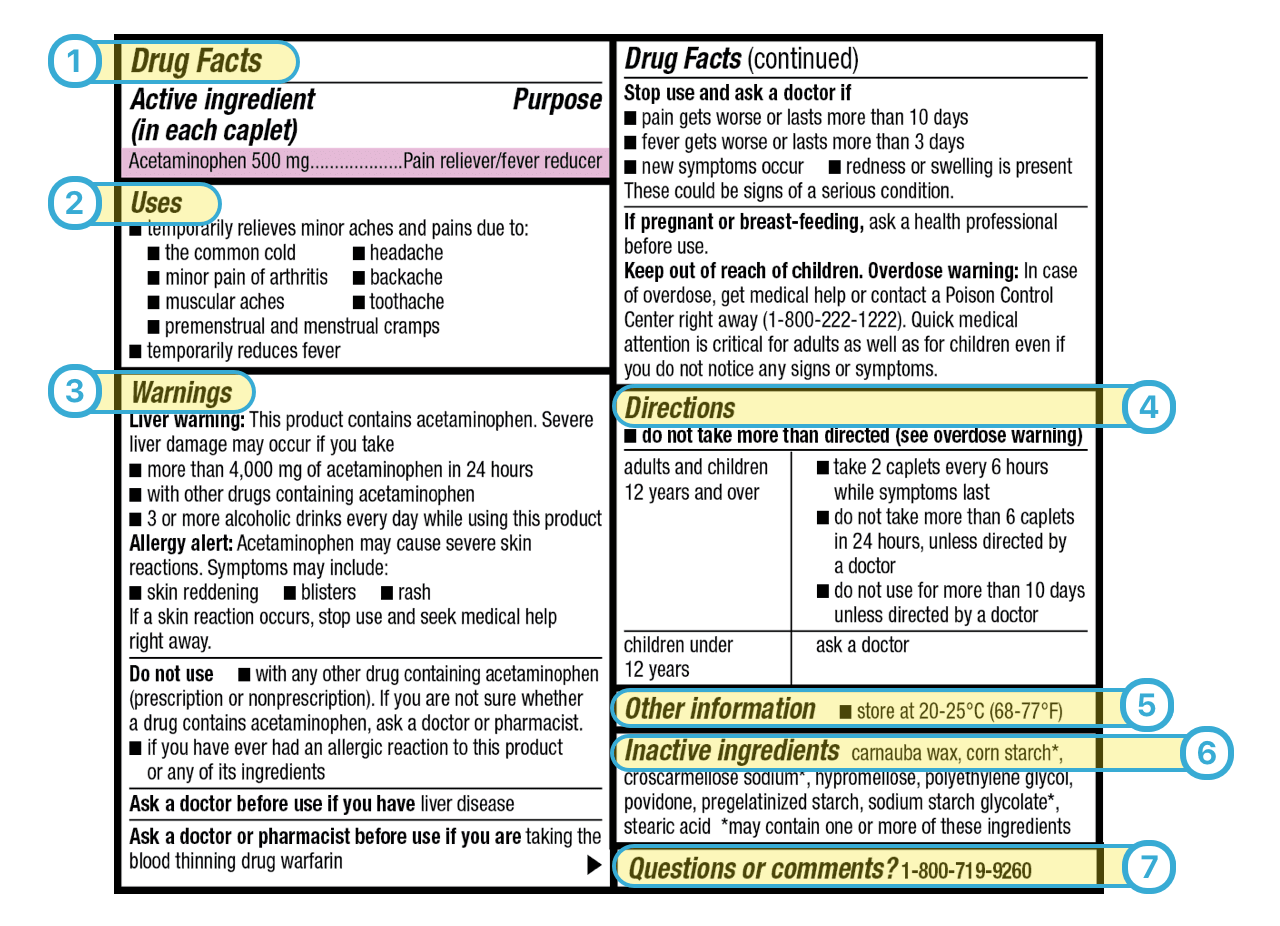

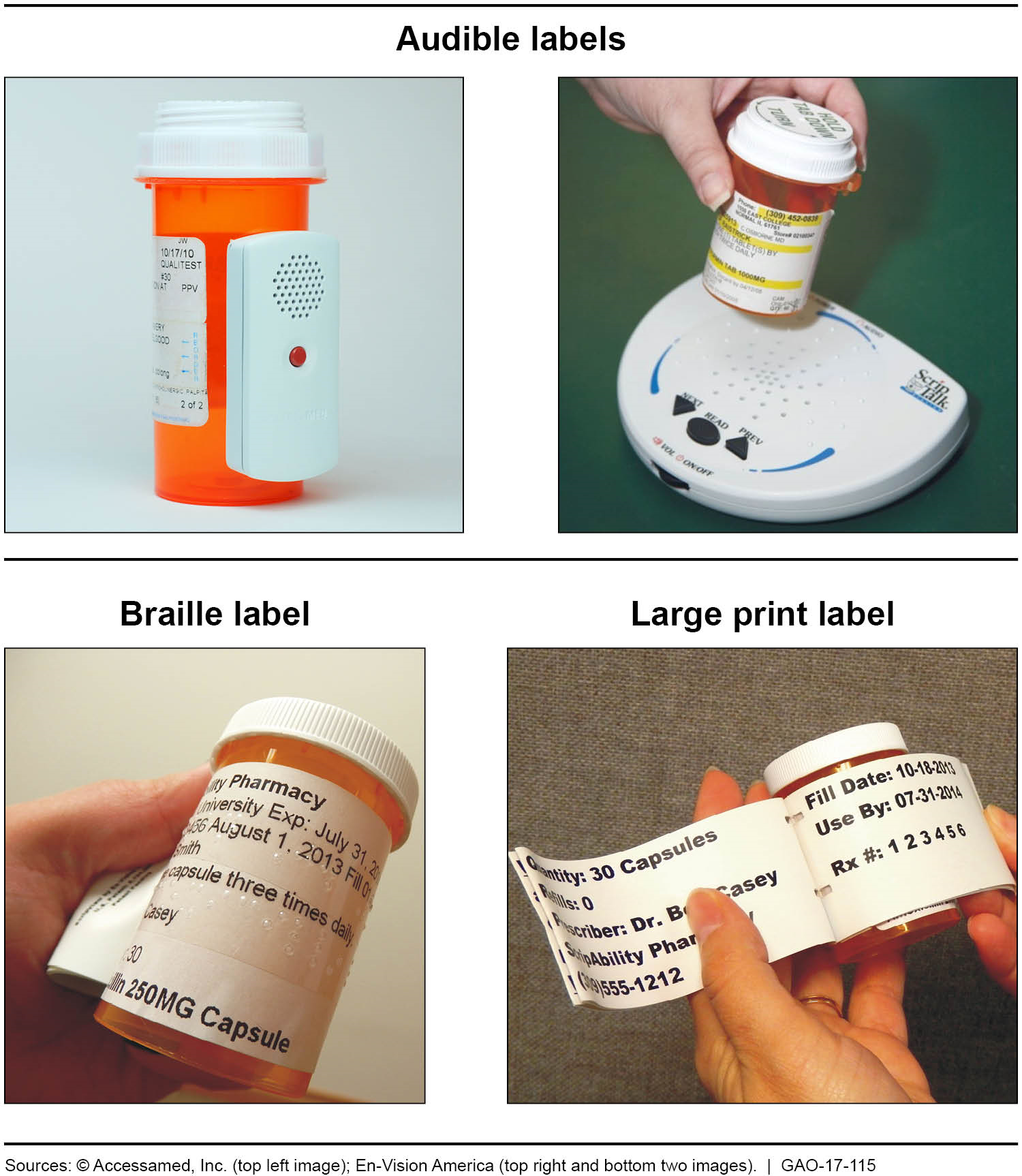


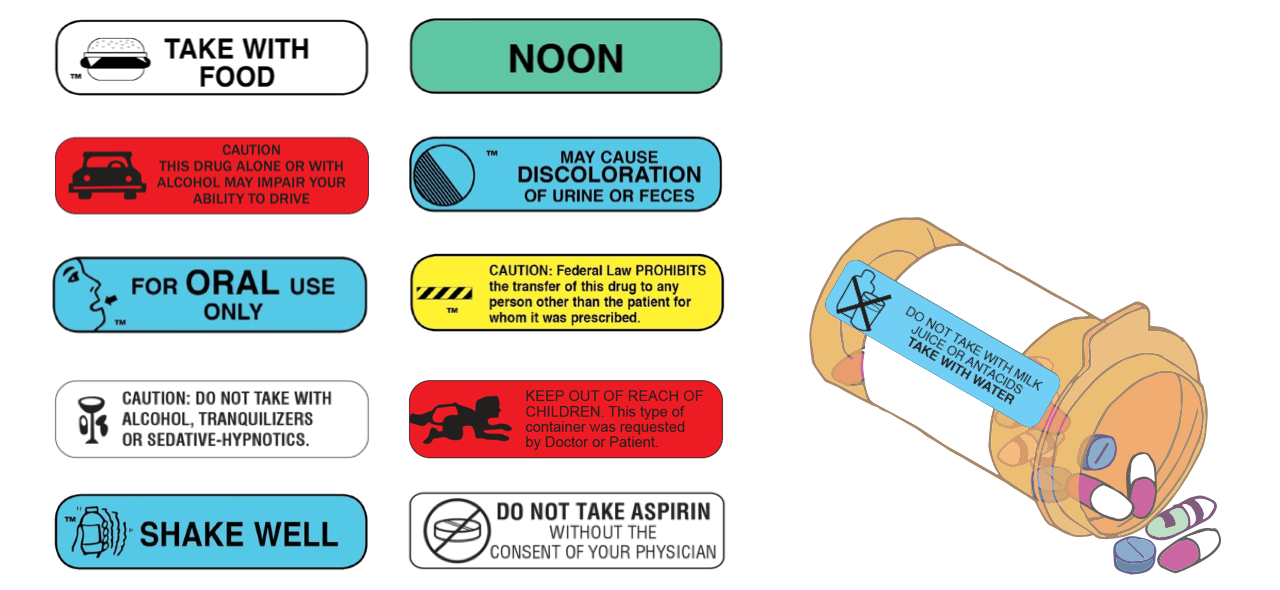













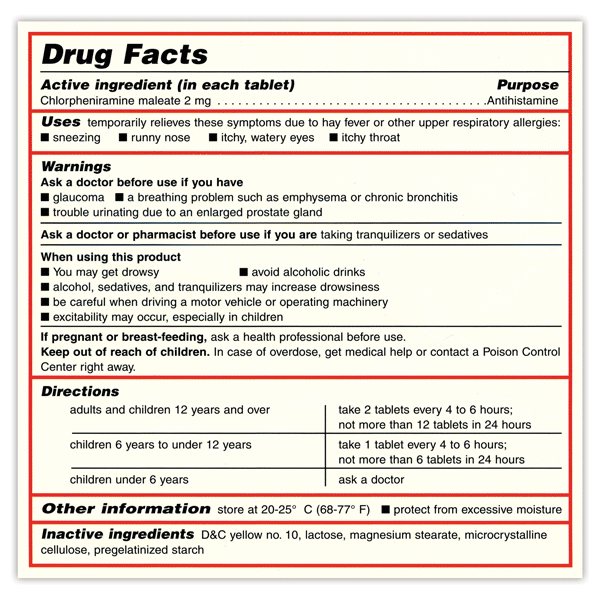

Post a Comment for "43 medication labels must include"